Women with breast cancer who receive aromatase inhibitors (AI) are at risk of bone mass loss and bone fracture. Many of these women have been previously treated with chemotherapy and/or tamoxifen, also with known deleterious bone effects. We studied the bone mineral status of a group of postmenopausal women with non-advanced breast cancer about to initiate AI treatment.
Between 2007 and 2010, 127 women aged 63±9 years were prospectively included. Clinical, epidemiological, analytical, radiological, and densitometric data were collected.
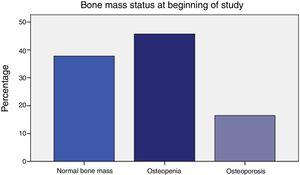
Prior to initiating AI treatment, the patients exhibited a high prevalence of bone mass loss (62.2%): 16.5% had osteoporosis and 45.7% osteopenia by OMS criteria. Besides, 7.4% of their dorso-lumbar spine x-rays revealed one or more vertebral fractures, all of which were found in patients with densitometry-defined osteoporosis or osteopenia. Surprisingly, 25-OH-vitamin D levels were normal (≥30 ng/ml) in 87.4% of the women. Obesity was prevalent (BMI 30±5). Their most common tumour was infiltrating ductal carcinoma (76.4%). The women who had received adjuvant chemotherapy (50%) were younger (59.1±8.3 vs. 66.2±8.2, p<.0001), and had greater total hip bone mass (.988±.138 vs. .935±.119, p=.048). The patients that had taken tamoxifen (37%) more often exhibited normal bone mass (55.3% vs. 27.5%, p=.009), and had greater bone mass at the femoral neck (.893±.113 vs. .826±.131, p=.003). In addition, they had lower bone turnover: lower osteocalcin (4.4±2.8 vs. 8.2±6.7, p<.0001) and deoxypyridinoline (7.1±2.4 vs. 8.3±3.3, p=.037).
This study shows that more than 60% of women about to initiate aromatase inhibitors for non-advanced breast cancer have low bone density and so high risk of fracture or re-fracture. These facts underscore the importance of screening their bone mass status and propose them preventive treatment.

