Background and objective
Systemic adverse effects (AE) are a major concern of low-dose oral minoxidil (LDOM) treatment, especially in patients with arterial hypertension or arrhythmia. The objective of this study was to evaluate the safety of LDOM in patients with hypertension or arrhythmia.
Patients and methods
Retrospective multicenter study of patients with hypertension or arrhythmia treated with LDOM for any type of alopecia.
Results
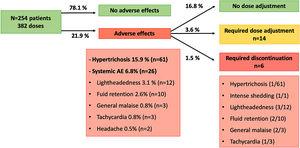
A total of 254 patients with hypertension [176 women (69.3%) and 78 men (30.7%)] with a mean age of 56.9 years (range 19–82) were included. From them, the dose of LDOM was titrated in 128 patients, allowing the analysis of 382 doses. Patients were receiving a mean of 1.45 (range 0–5) antihypertensive drugs. Systemic AE were detected in 26 cases (6.8%) and included lightheadedness (3.1%), fluid retention (2.6%), general malaise (0.8%), tachycardia (0.8%) and headache (0.5%), leading to LDOM discontinuation in 6 cases (1.5%). Prior treatment with doxazosin (P<0.001), or with three or more antihypertensive drugs (P=0.012) was associated with a higher risk of discontinuation of LDOM.
Conclusions
LDOM treatment showed a favorable safety profile in patients with hypertension or arrhythmia, similar to general population.