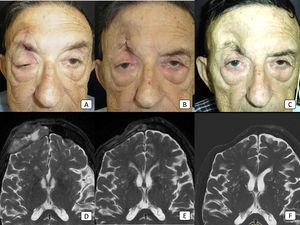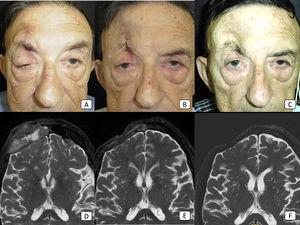
Management of advanced cSCC is challenging, and many available systemic medications have modest efficacy. Cemiplimab has demonstrated efficacy in the treatment of advanced cSCC in clinical trials, but real-world data are still limited. With the objective of evaluating the efficacy of cemiplimab in a real-world clinical setting, we conducted a prospective observational study of 13 patients with advanced cSCC. Six patients (46%) had locally advanced disease, while 7 (54%) had metastatic disease. A total of 8 patients (62%) responded to cemiplimab. Five (38%) showed a partial response, while 3 (23%) showed a complete response. Four patients with an initial partial response presented subsequent disease progression during follow-up. Six patients (46%) developed AEs, most of which were mild (G1). PFS was 5.9 months, with a median follow-up was 9 months. In conclusion, cemiplimab demonstrated its utility in the treatment of advanced cSCC, with acceptable response rates, a remarkable number of complete responses, and a very good safety profile.