Introduction and objective
Teriflunomide is an oral immunomodulatory agent approved for the treatment of relapsing–remitting multiple sclerosis (RRMS). We examined teriflunomide outcomes in patients with RRMS under clinical practice conditions in Spain.
Material and methods
Non-interventional, retrospective study at 15 sites in the Autonomous Region of Madrid and nearby regions. Effectiveness (relapses, EDSS, gadolinium-enhancing T1 lesions and new/enlarged T2-weighted lesions), safety (adverse events), and reasons for discontinuation during the 24 months after teriflunomide initiation were reported.
Results
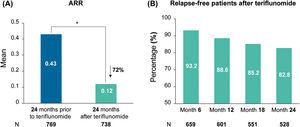
A total of 776 patients were included (mean [SD] age was 43.3 (9.8) years; 69.3% were female). Two-thirds (67.7%) of patients had received a prior treatment, with beta-interferons or glatiramer acetate (BRACE) as the most frequent (93.5%) treatment. After 24 months, teriflunomide significantly reduced the annualized relapse rate (ARR) by 72% (mean [95% confidence interval] 0.12 [0.10, 0.14] vs 0.43 [0.40, 0.47] at baseline; P<.0001) and 81.8% of patients were relapse-free. The number of patients with gadolinium-enhancing T1 lesions and new/enlarged lesions on T2 was also reduced after 24 months of teriflunomide treatment (P<.001). Mean EDSS (SD) score was 1.9 (1.5) at teriflunomide initiation and 2.0 (1.6) at month 24. Half of patients (n=388) reported at least 1 adverse event (AE; gastrointestinal disorders: 26.2%; hair thinning: 25%; and elevation of ALT values: 12.9%). Most patients (91.5%) did not show fatigue increase during teriflunomide treatment. Among patients who discontinued treatment (n=262; 34.2%), the most common reasons were lack of effectiveness (58.0%), AEs (31.9%; n=82), and pregnancy desire (6.6%; n=17).
Conclusions
Most RRMS patients treated with teriflunomide in clinical practice had received prior treatments. Teriflunomide resulted in decreased clinical and radiological activity and disability stabilization. AE frequency and type were in line with prior reports. Most patients did not experience fatigue increases after teriflunomide initiation.


